 Not actual patient.
Not actual patient.
LET’S TALK ABOUT
PATIENTS LIKE JASON
A patient with type 2 diabetes struggling to maintain glycemic control
Diagnosed with type 2 diabetes 6 years ago
On daily regimen of multiple oral agents (OAs), weekly GLP 1 injection, and just started using CGM
Has an A1C over 9.0 and wants to achieve glycemic target
Doesn't want to deal with daily mealtime injections
Helps Jason
identify glucose
excursions
GIVE PATIENTS LIKE JASON IMPROVED MEALTIME CONTROL WITH AFREZZA®1,8
- Afrezza delivers an ultra-rapid insulin response with absorption in the blood in <1 minute1,4
- Time to first measurable effect is ~12 minutes1
- Patients inhale Afrezza at mealtime, when they are ready to eat, with no needlesticks1
- One out of three patients with type 2 diabetes treated with OAs plus Afrezza achieved
A1C ≤7% versus OAs alone1
 Not actual patient.
Not actual patient.
Patient Profile:Jason
Drag & Drop:What features of an inhaled insulin might help a patient like Jason?
Drag the items to sort them in the order of importance to you, with 1 being the most important
A1C levels1,8

Jason’s
Journey
ranking
LET’S CHANGE THE CONVERSATION WITH
SUPERIOR CONTROL
Adding Afrezza® significantly reduced A1C levels compared to OAs alone1,8
APPROXIMATELY ONE OUT OF THREE PATIENTS ACHIEVED A1C ≤7% WITH AFREZZA1
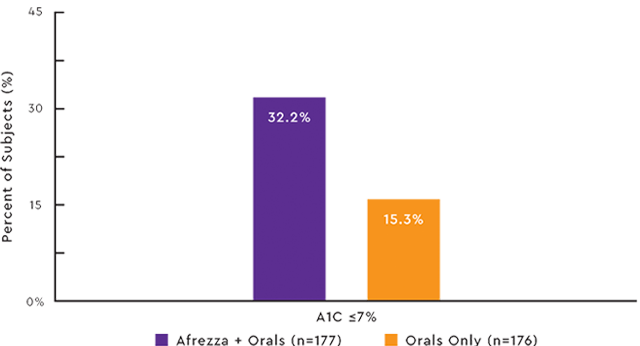

Study design:Afrezza efficacy was studied in a 24-week, double-blind, placebo-controlled, international, multicenter phase 3 trial of insulin-naive adults with type 2 diabetes (n=353) uncontrolled (A1C >7%) on optimal/maximally tolerated doses of either metformin alone or 2 or more OAs. Patients were treated with Afrezza plus OAs or inhaled placebo powder without insulin plus OAs.
The primary efficacy endpoint was the average change in A1C from baseline (randomization) to week 24. At week 24, Afrezza plus OAs had an A1C of -0.82%, OAs only had an A1C of -0.42% (P<0.0001).8

Jason’s
Journey
ranking
LET’S CHANGE THE CONVERSATION WITH
SUPERIOR CONTROL
Adding Afrezza® significantly reduced A1C levels compared to OAs alone1,8
GIVE YOUR PATIENTS WITH TYPE 2 DIABETES IMPROVED MEALTIME CONTROL1,8

ADDAfrezza to OAs to avoid introducing injections1,8

ADDAfrezza to basal insulin to avoid mealtime injections10


Jason’s
Journey
ranking
LET’S TALK ABOUT
THE SAFETY PROFILE
INCIDENCE OF SEVERE AND NON-SEVERE HYPOGLYCEMIA IN A PLACEBO-CONTROLLED TYPE 2 STUDY1
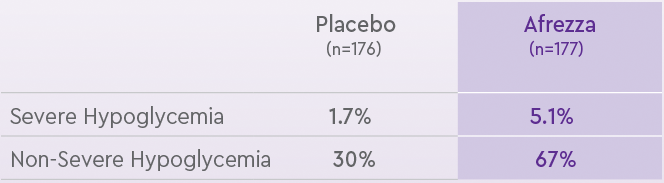
Study design: Afrezza efficacy was studied in a 24-week, randomized, double-blind, placebo-controlled, international, multicenter phase 3 study in insulin-naive adults with t2dm (n=353) uncontrolled (A1C > 7%) on optimal/maximally tolerated doses of either metformin alone
or 2 or more OAs. Patients were treated with Afrezza + OAs or inhaled placebo powder without insilin + OAs.

Jason’s
Journey
ranking
LET’S TALK ABOUT
THE SAFETY PROFILE
Afrezza® has been studied in over 3,000 patients with diabetes1
MOST COMMON ADVERSE REACTIONS (EXCLUDING HYPOGLYCEMIA) FROM POOLED
SAFETY RESULTS IN TYPE 2 DIABETES1
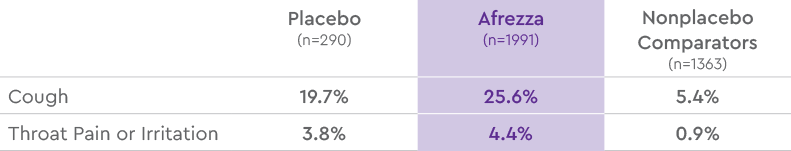
Common adverse reactions, excluding hypoglycemia, associated with the use of Afrezza in the pool of controlled trials in type 2 diabetes patients. These adverse reactions were not present at baseline, occurred more commonly on Afrezza than on placebo and/or comparator, and occurred in at least 2% of patients treated with Afrezza.1

Jason’s
Journey
ranking
LET’S CHANGE THE CONVERSATION ABOUT
DOSING + TITRATION
Afrezza® mealtime control is possible with flexible dosing1
12 - 24 Units
Typical mealtime dose in clinical trials7,9
1.5x
Suggested conversion from injectable insulin to Afrezza units for comparable effect7,9,10
Adjust Dosing
Example: Increase by 4 units per meal every 3 days until glucose is controlled
This Afrezza Titration Pack offers flexibility for patients getting started:


Jason’s
Journey
ranking
LET’S CHANGE THE CONVERSATION ABOUT
DOSING + TITRATION
Afrezza® mealtime control is possible with flexible dosing1
EXAMPLE DOSING FOR PRANDIAL INSULIN NAIVE
4 Afrezza Units
given with each meal for 3 days
(total of 12 Afrezza units per day)

Dose for 3 days
Dose adjusted to 8 units with each meal based on 2 hr PPG x 3 days (PPG >160 mg/dL)
8 Afrezza Units
given with each meal for 3 days
(total of 24 Afrezza units per day)

Titrate up to 3 days
Dose adjusted to 12 units with each meal based on 2 hr PPG x 3 days (PPG >160 mg/dL)
12 Afrezza Units
given with each meal for 3 days
(total of 36 Afrezza units per day)

Titrate to effect
Dose adjusted to 16 units with each meal based on 2 hr PPG x 3 days (PPG >160 mg/dL)

every 3 days until PPG is controlled.


