LET’S CHANGE THE CONVERSATION BY
UNDERSTANDING LIMITATIONS
Injectable insulin is effective—but complexities limit its use6
PHYSIOLOGIC CONTROL
Currently, there is a lack of insulin
therapies that match the time action profile of physiologic insulin11
TREATMENT COMPLEXITIES
Challenges of coordinating timing of mealtime injections and injection burden all limit traditional insulin use6,11
DELAYED INITIATION
The initiation of traditional insulin therapy is often delayed in patients with type 2 diabetes12
POOR COMPLIANCE
As many as 1 in 5 mealtime injections
are intentionally missed6
LET’S CHANGE THE CONVERSATION WITH
A UNIQUE PROFILE
Afrezza® has a time action profile that mimics physiologic insulin13
PHYSIOLOGIC POSTPRANDIAL INSULIN PROFILE5
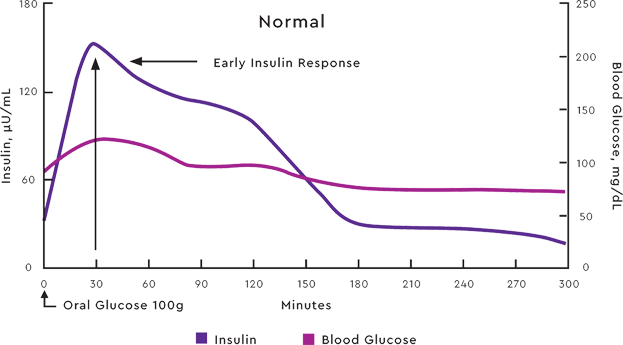
Adapted from: Boli GB, etal. N Engl J Med.1984;310:1706-1711. Ciofetta M, et al. Diabetes Care. 1999;22:795-800.
LET’S CHANGE THE CONVERSATION WITH
A UNIQUE PROFILE
Afrezza® has a time action profile that mimics physiologic insulin13
AFREZZA POSTPRANDIAL INSULIN PROFILE1
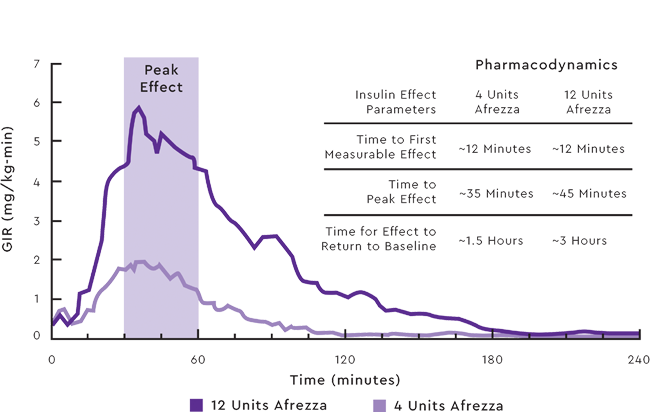

Data from a randomized, controlled, six-treatment, crossover
dose-response study compared Afrezza with the rapid-acting insulin analog, lispro, in 30 patients with type 1 diabetes. Mean insulin effect (baseline-corrected glucose infusion rate) profile shown after administration of 4 and 12 Afrezza unit doses in patients with type 1 diabetes. On average, the pharmacodynamic effect of Afrezza, measured as area under the glucose infusion rate—time curve (AUC GIR)—increased linearly with doses up to 48 units. Intrapatient variability in AUC GIR and GIRmax is approximately 28% (95% CI 21-42%) and 27% (95% CI 20-40%), respectively.1
LET’S CHANGE THE CONVERSATION BY
CONSIDERING THE PATIENT
Afrezza® mealtime control should be considered in the context of weight change1,7,8
WEIGHT PROFILE IN 2 PIVOTAL TRIALS1,7,8
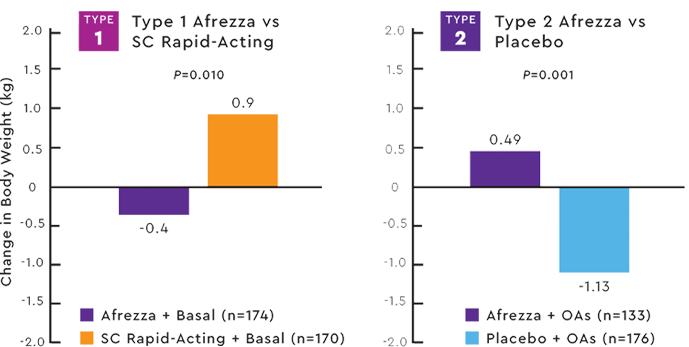
 Study design: (type 1):Open-label
Study design: (type 1):Open-label
non-inferiority trial comparing the change in A1C baseline to week 24 of prandial Afrezza with SC rapid-acting insulin, both with basal insulin in adult patients with type 1 diabetes and an A1C >7.5%.
Patients on Afrezza lost weight (-0.4 kg) compared with weight gain (+0.9) for patients on SC rapid-acting insulin (P=0.0102). Afrezza and basal insulin was therefore considered non-inferior to SC rapid-acting and basal insulin in terms of less weight gain.7
Study design (type 2):Double-blind placebo-controlled trial in patients with type 2 diabetes with an A1C ≥7.5% on metformin alone or 2 or more oral agents (OAs) randomized to receive prandial Afrezza (n=133) or prandial inhaled placebo (n=176).
Over the 24-week treatment period, there was an average weight increase of 0.49 kg in the Afrezza group compared with a weight loss of 1.1 kg in the placebo group.8
LET’S TALK ABOUT
THE SAFETY PROFILE
Afrezza® has been studied in over 3,000 patients with type 1 and type 2 diabetes1
INCIDENCE OF COUGH IN POOLED SAFETY POPULATION1,9
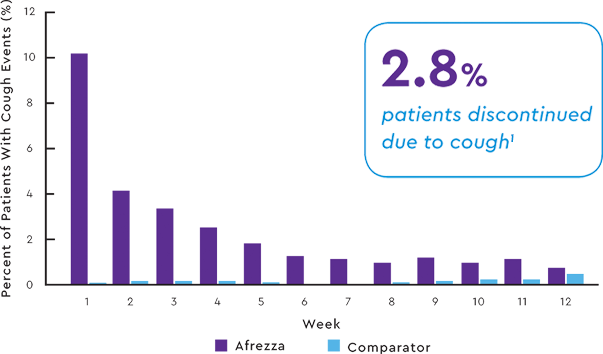
A CLOSER LOOK AT COUGH
- 94% of cough episodes were characterized
as intermittent or single defined episodes9 - Cough was generally mild, dry, occurred within 10 minutes of inhalation, was transient and declined with continued use9
LET’S TALK ABOUT
ACCESS + SUPPORT
MannKind is committed to helping patients access Afrezza®

Pay as little as $15 per
Afrezza prescription.*
Afrezza Savings Card
Patients with commercial insurance may pay as
little as $15 for each prescription, even if insurance denies or requires a prior authorization.
Patients can download the Savings Card at
www.afrezzasavingscard.com
*Eligibility criteria and maximum benefit limitations apply.
See terms and conditions for complete details.
ADDITIONAL OPTIONS FOR
ALL OF YOUR PATIENTS
Additional programs and services may
be available for your patients who are
not eligible for the Savings Card.
For details, call the MannKind Customer
Experience Center at(833) 623-4843or emailguide@mannkindcorp.com
LET’S CHANGE THE CONVERSATION BY
GETTING STARTED
Improved mealtime control starts with gaining meaningful experience with Afrezza®
in your practice
START WITH 5 PATIENTS
Identify 5 type 1 or 2 patients who would
benefit from improved mealtime control
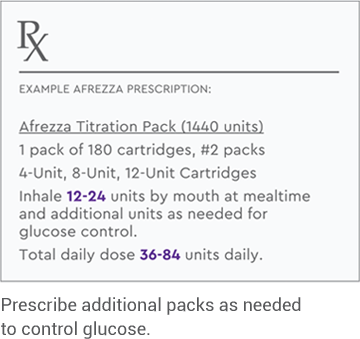
PRESCRIBE AFREZZA
Select the right dosing option
for each patient
SEE IMPROVED MEALTIME CONTROL
See the impact Afrezza may have on your
patients' glycemic control at their next visit
IT’S TIME TO CHANGE THE CONVERSATION AROUND
MEALTIME CONTROL
Give your patients improved mealtime control with Afrezza®1,2
FAST IN, FAST OUT1,4
Time to first measurable effect is ~ 12 minutes.
In the blood in <1 minute.
Peak effect in 35-45 minutes.*
Out within 1.5-3 hours.*
MANAGEMENT IN THE MOMENT1
Inhale when food arrives.
Option to take more if needed.
Mealtime flexibility.
CONSIDER THE PATIENT1,7,8
No mealtime needlesticks required.
Up to 1,000 fewer injections per year.†
Weight-neutral profile.
PROVEN MEALTIME CONTROL1
Over 3,000 patients studied.
Both type 1 and type 2 diabetes.
*Based on 4-12 unit dosing, respectively. On average, the pharmacodynamic effect of Afrezza, measured as area under the glucose infusion rate—time curve (AUC GIR)—increased
linearly with doses up to 48 units.1
†Based on an estimation of approximately 3 meals per day, for a total of 1,095 possible mealtime injections per year.
IT’S TIME TO CHANGE THE CONVERSATION AROUND
MEALTIME CONTROL
Thank you for your participation!
We hope you found this Interactive Learning Module on mealtime control informative and engaging.
Request a Rep+Educational Materials→



